External Alignment
In order to fulfil the 1+MG ambition, the 1+MG Framework collects the recommendations and guidelines of EU experts to facilitate access to genomic, phenotypic and other types of data across borders. The 1+MG Framework therefore can be used by the European Health Data Space (EHDS) when tackling the access to genomic data for secondary use. Most processes, standards and reference implementations related to the management of genomic information could be used by 1+MG, EHDS and other initiatives and projects that would need to share access to genomic information across borders, ensuring technical interoperability.
TEHDAS Data Lifecycle
This section outlines the elements of the Data Lifecycle as defined by the TEHDAS joint action. It includes a description of each stage, guidance, standards, and other relevant considerations.
This cycle has gone through multiple iterations by TEHDAS and we examine the connection of the 1+MG Framework with the proposed Data Lifecycle as of June 2023.
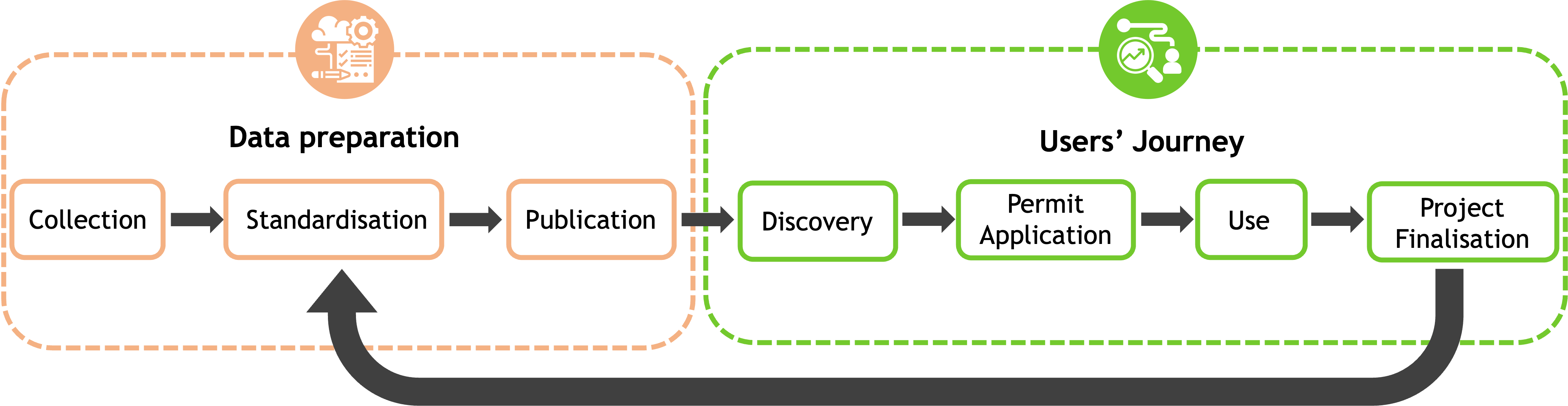
 |
 |
||
| Data Preparation | Collection | Data Quality & Inclusion | Governance & ELSI |
| Standardization | Data Models & Ontologies | ||
| Data Reception | |||
| Publication | Data Storage & Management | ||
| Users' Journey | Discovery | Data Discovery | |
| Permit Application | Data Access Management | ||
| Use | Data Processing | ||
| Project Finalization | |||
The above table lists the stages of the TEHDAS Data Lifecycle (which encompases both the Data Preparation phase and the User Journey phase) and the associated elements of the 1+MG Framework. The 1+MG Framework Governance and ELSI recommendations are relevant across the Data Lifecycle.
There are some elements that fall outside of this mapping. The TEHDAS Project Finalization stage is related to the disclosure of results, which currently falls outside of the scope of 1+MG’s technical recommendations, but there are some relevant ELSI policies, such as the forthcoming “Policy on incidental findings” and the “Policy for communication of general research results”. Additionally, there are some elements of the 1+MG Framework which fall outside of the TEHDAS Data Lifecycle including the Implementation of Genomics into Healthcare resources (focused on supporting national efforts at using genomic data within personalised medicine) and the specific scientific Use Case resources.
We are still working on the content for this page. If you are interested in adding to the page, then:
This is a community-driven website, so contributions are welcome! You will, of course, be listed as a contributor on the page.