Healthcare integration and sustainability
The implementation of genomic medicine in healthcare systems will bring us closer to making personalised medicine a reality, with major socioeconomic benefits.
Citizens and patients can nowadays widely benefit from genomic data analysis for accurate and timely diagnosis, more effective treatments with fewer adverse events and, in the near future, accurate profiling for disease prevention.
Implementation of genomics in healthcare is complex and requires adjustments in the governance, structure and organisation of health services, as well as dedicated investments. Moreover, the progress of implementation varies with country context.
Facilitating dialogue and cooperation among countries for capacity building and sharing of best practices is key to promoting equity in access to Personal Medicine across Europe.
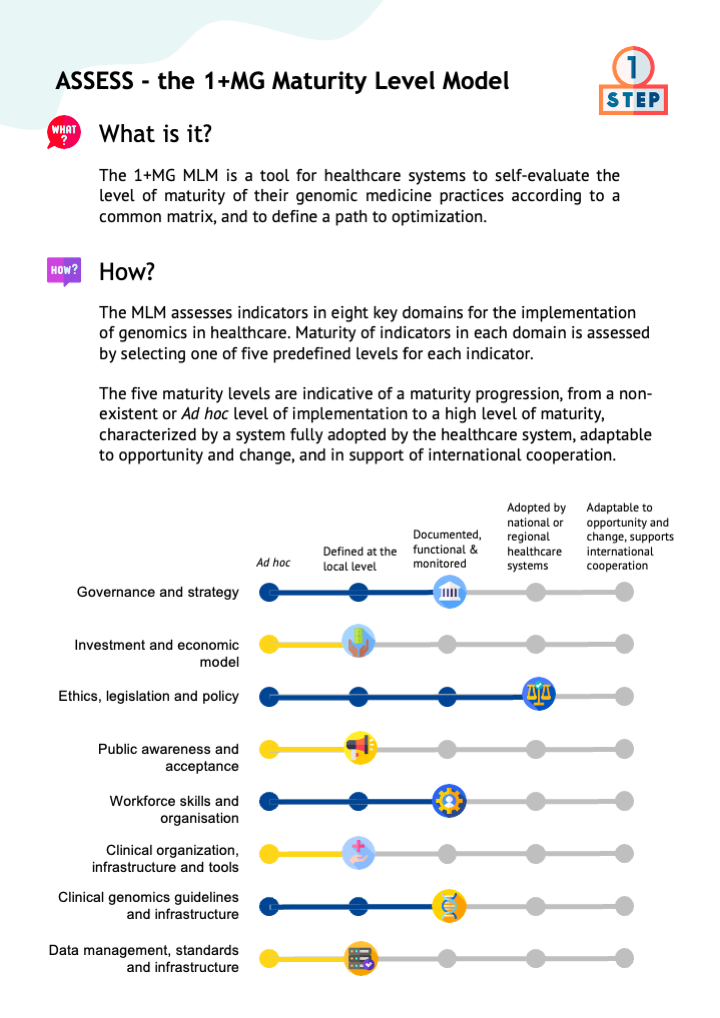
To fully exploit the potential of genomic information to benefit citizens health, it is crucial to understand how effectively healthcare systems implement genomic medicine. In the context of the 1+ Million Genomes Initiative (1+MG), a Maturity Level Model (MLM) was developped for healthcare systems to evaluate the maturity of their genomic medicine practices, and define a path to optimization.
Maturity Level Model
Genomics in Healthcare Maturity Level Model
A tool for healthcare systems to self-evaluate the level of maturity of their genomic medicine practices according to a common matrix, and to define a path to optimization.
The MLM assesses indicators in eight key domains for the implementation of genomics in healthcare. Maturity in each domain is assessed by selecting one of five pre-defined maturity levels regarding the specified indicators.
The five maturity levels are indicative of a maturity progression, from a non-existent or Ad hoc level of implementation to a level of maturity characterized by a system adaptable to opportunity and change, and in support of international cooperation.
A 1+MG MLM for Healthcare User Guide is available
B1MG Maturity Level Model Report
The Genomics in Healthcare Maturity Level Model (GiH MLM) was created as a tool for countries to self-assess the maturity level of implementation of genomics into their healthcare systems, according to a common matrix, and to define a path to optimization. As such, it aims to promote and facilitate the adoption of genomics in healthcare systems, close the best practice gaps across Europe, and make personalised medicine accessible to citizens and patients across Europe.
This report describes the design of the 1+MG MLM for Healthcare and the content validation through a Delphi exercise.
Roadmap and Guidance Tool
The Roadmap Infographic and Guidance Tool is available for countries that support the 1+MG B1MG Maturity Level Model for Healthcare.
This roadmap is a very comprehensive guide to the current status of the field of genomic medicine in healthcare, covering all crucial areas with the most up to date recommendations for genomic medicine in healthcare.
1+MG Framework
You are here.
Policy Brief
Genomics in Healthcare: Key issues for implementation Recommended
To achieve the goal of implementing genomics in healthcare systems, countries need to establish national genomic medicine strategies. However, it is clear that European countries are currently at varying stages of maturity for using genomics in healthcare. Promoting the dialogue and cooperation among countries, for capacity building and sharing of best practices, is therefore extremely beneficial for advancing genomic medicine at the national and European levels. This policy brief discusses some key issues to build efficient and sustainable genomic medicine strategies such as:
- Patient and citizens trust and engagement
- Infrastructure for implementation of genomics in healthcare practice
- Ethical and legal frameworks
- Synergies among healthcare, research and industry
- Training of healthcare professionals
Country Visits Informational
As part of the B1MG project, we virtually visited multiple countries in Europe to unveil their genomics infrastructure. Between March and June, we visited the UK, Estonia and Finland. You can now (re)play all talks from these successful, international meetings.
Health Economic Outcomes Research
As part of the B1MG Project and together with partners of the 1+MG initiative, several activities regarding Health Economics and Outcomes Research (HEOR) were done. Two workshops and other meetings resulted in the following three publications:
1+MG HEOR Summary Brief
This brief contains a discussion about the status of EU initiatives to foster the evaluation and implementation of whole genome sequencing into the clinical practice in the Member States.
1+MG HEOR Workshop Summary
This documents contains the results of a workshop dedicated to develop recommendations towards the Member States and the European Commission with the purpose to advance in the development of useful economic evaluation methods applicable to genomics.
B1MG Economic models methodology
This is a report which has the results of a workshop dedicated to analyse Health Technology Assessment and Health Economic models applicable to genomics. The objective of the workshop was to provide insights and facilitating discussion on experiences of national genome initiatives and/or relevant projects from Canada, France, the Netherlands and the United Kingdom. The document analyses those national experiences and discussed lessons learned. In addition identifies some key challenges for a successful application of the Health Technology Assessment and Health Economic methods to genomics. Finally provides a series of key recommendations, some related to the role of HTA in genomics, others with methodology and others about opportunities for international collaboration.